Order of a reaction
Order of a reaction: Overview
This topic covers concepts, such as Order of a Reaction, Zero Order Reactions, Second Order Reactions and First Order Reactions.
Important Questions on Order of a reaction
The half-life for decay of radioactive is 5730 years. An archaeological artifact containing wood has only 80% of the activity as found in living trees. The age of the artifact would be:
[Given: log 1.25 = 0.0969]
For the following elementary reaction, determine its order of reaction and the dimensions of the rate constant:
For a reaction, , the rate is given by , hence, the order of the reaction is:
For the reaction product
order of reaction is :-
The rate of the first order reaction products is , when reactant's concentration is . The rate constant for the reaction will be
The slope of the straight line obtained by plotting rate versus concentration of reactant for a first order reaction is.
The unit of rate constant for first order reaction is
Statement : The exponents for concentration do not necessarily match the stoichiometry coefficients in the rate law expression.
Statement : In the rate law expression, exponents for concentration are determined by experiments not by balanced chemical reaction.
is a first order reaction.
If the initial pressure is and the total pressure at time 't' is , then the rate constant is:
Find out the order for the given reaction
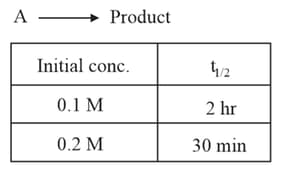
Calculate the half life for a zero order reaction, if reaction is completed in .
What is the correct representation of graph for a reaction of first order kinetics (A: moles of reagent remaining, t: time)?
Radioactive decay is an example of which order of reaction?
Calculate the overall order of the reaction, given that a reaction involving A, B and C as reactants is found to obey the rate law, rate=. When the concentration of A, B and C are doubled separately, the rate is also found to increase two, zero and four times respectively.
For a first order reaction,
Reaction progress is measure with the help of titration of reagent 'R'. If all reacted with reagent and have 'n' factors in the ratio of 1 : 2 : 3 with the reagent. Calculate k in terms of t, and :
How much time will it take for 99.9% completion if 75% completion of a first order reaction takes 2.38 hours?
The graph which is/are correct for a first-order reaction is:
If for the reaction is minutes. Then, calculate the time required for the completion of a order reaction.
If the time required to complete of reaction , which is the same as of reaction and the first-order reactions are given below, then find the ratio of the rate constants ().
